
Richard Schneider has dedicated over 36 years to the pursuit of Quality Assurance. His experiences include multiple long-term roles as a Multi-Plant Quality Director, a Supplier Development Leader for a large OEM in Asia, and extensive supplier development travels in mainland China. He mentors quality professionals and is a student of the Chinese Mandarin language.
I’ve witnessed smart, driven people suffer from gaps in their quality processes because of limitations in their tools. They knew what objectives mattered, and their QMS perfectly addressed the requirements of the standard, but these teams lacked a simple and viable digital model encompassing the entire documentation process: from the review of customer requirements, supplier flow-down and validation, internal manufacturing inspection, data analysis, to customer reporting.
They had a patchwork of Word and Excel templates, an ERP loaded with product details, calibration software, and an SPC package. All of this had to come together into Control Plans, FMEA’s, First Articles, Control Charts, and Material Certifications. This would need to be compiled into a packet that had to be flawless and current every time. Multiply this effort times the number of active part numbers in the system, and the frequency of revisions changes, and the result is a never-ending paper chasing exercise, driven by the shipping schedule. With hybrid systems not enough people know how they work, and therefore too few people are able to troubleshoot if a problem arises. But it’s not all bad news; I’ve helped many companies improve these processes and gain control of their quality reporting processes.
Scenario: Ship List Reveals a Revision
A typical scenario: the month-end ship list reveals a pending delivery for a new part or revision change. A QA employee opens the First Article report, often the shared Excel file that needs an update. The report is missing data so they open another Excel file with measurement records, copy and paste data, then access a DOS system for some header information, jot a few notes on a piece of scrap paper, and transcribe them to the report. With so much copy/pasted and transcribed information, typos are inevitable. Sometimes there isn’t enough time to finish the reports before shipping, the deadline is too tight. So the company ships the parts anyways, then sends the reports just as the parts reach their destination. Leveraging transit time to complete paperwork is a desperate QA manager’s last hope. Occasionally a dimensional nonconformance is discovered in the compiling process. This is when the risk, true cost, and inefficiency of these tangled hairball of systems is exposed.
The customer complains, the Plant Manager blames the QA manager, the QA manager laments the shortcomings of the system, but fixing such a complex problem feels like trying to solve world hunger. In the end, another crisis arises, QMS discussions taper off, and nothing changes, only to repeat the whole ugly process again next month.
In this environment the Quality Manager blames the lack of resources and the Plant Manager blames the Quality Manager for not working on the right things at the right time. When faced with tight timelines, limited resources, and no holistic tools for a seamless execution, the QA manager is overworked, burns out fast, or gets forced into early retirement, and a new person is ushered in to face the same problems. Hopes are high, but not for long.
To Address Systemic Issues Improve Whole Processes
Trade shows are packed with software and hardware solutions designed to speed up and automate the processes, and individually many appear to address various specific issues effectively. Quality Managers report back to their management proposing some hardware or a new software program that appears to offer improvement to one of the component problems. A faster CMM, a data migration tool, or an electronic print numbering software which eliminates legibility problems, but it all gets turned away by upper management. Either it is too expensive, or the company has a history of buying software they don’t implement. So nothing changes and again, missed deadlines continue to come and go with no real improvement to the system.
I’ve been immersed in these same problems since I started in quality, and I’ve developed some good incremental process changes that I considered solutions, but these never resulted in a truly seamless system. Sure, I was viewed as a success, and I repeated similar improvements in several companies, but eventually I concluded that my incremental improvements and partial systems were evidence that nobody was addressing this problem as a whole. I started to see ERP systems that tried to solve this problem, but their quality modules were not designed by someone who understood the quality field. These ill-designed fixes just complicated the problem even more. So I continued to roll out improvements, making a good career out of solving problems with incremental changes, and did so amazed, and often frustrated, that somebody had not resolved what was surely an industry-wide dilemma. That all changed when I discovered that InspectionXpert not only understood the problem, but had built a solution.
InspectionXpert develops a software program that at first glance, presents itself as print numbering software. It does this well, but what most people seem to overlook (or forget) somewhere after seeing the demo and before seeking purchase approval, is the much broader application it offers. It creates a holistic unity spanning the documentation required between accepting the drawing and shipping the parts. InspectionXpert is able to translate and export every drawing field (dimensions, notes, title block information), into any company custom report, industry standard, or regulatory format. In the end, whatever the organization’s document requirements are, if a basic template is created, InspectionXpert will populate it.
What does this look like in execution? You import a PDF print then number it electronically by clicking each drawing note or dimension. Now you are able to generate and output all of the documents with the click of the mouse. Depending upon the size of your organization, you just eliminated one or more of the full-time administrative positions dedicated to Quality Assurance configuration management.
How I Used InspectionXpert to Decrease Non-Conformances From Suppliers by 30%
I deployed InspectionXpert as I took on new positions, and I always landed these roles, in part, because of the innovations I promised to deploy using InspectionXpert. Each deployment was more successful than the last as I deepened my understanding and refined my methods. The most rewarding was the time I deployed it throughout a well-known OEM and their global supply base. I was the Staff Engineer responsible for safety critical systems and tasked with mentoring the SQE team in all things related to quality assurance. Our employer had experienced poor quality from the supply chain, resulting in unprecedented product recalls, and needed a new approach to reducing defects in the supply base. 80% of their component suppliers were located in Low Cost Countries and they lacked a systemic method of flow-down and validation of externally supplied products.
I created a planning team and we developed a supplier monitoring process that required our SQE’s over each commodity to work with design to identify key characteristics for their high cost/high risk parts and assemblies. We then developed KPC’s (Key Product Characteristics) and sampling plans, but needed a mechanism to flow these down to the supply base. The timing was right for me to call upon my experience and understanding of the InspectionXpert software program to facilitate, standardize, and automate this process. I had the entire SQE team download the free trial of InspectionXpert, and trained them so we could standardize inspection documentation. The process was a 3 phase program:
We developed standardized supplier quality reports that would communicate the measurement results to be supplied with each shipment. We established a 3rd party inspection lab where product ship lots were routed for validation before delivery to our assembly plants. The plan followed the ANSI/ASQ Z1.4 sampling standard, applying an appropriate AQL until 10 consecutive lots passed, with reduced inspection and eventually skip lot inspection was enacted for high performing suppliers. Our factories began to report a reduced level of defects.
We discovered that as soon as we sent the supplier a formal notice that we would begin monitoring their parts and that we would charge them for the use of a third party to sort for any nonconformances found, there was about a 30% reduction in the PPM on the subsequent shipments. This revealed that the mere threat of using a 3rd party resulted in some suppliers taking effort to ship better quality. Yes, we knew that some suppliers would play it smarter now that a dollar value was placed on rejections, but nobody really expected such a sharp reduction in defects.
But even more significant was the reduction in defects that occurred once we actually started to route shipments through our 3rd party lab and charge suppliers whenever a lot failed an AQL sampling. Even the chronically worst suppliers suddenly became markedly better when their profit margin was eroded with a high hourly sort fee. The chart below shows how these three phases impacted quality.
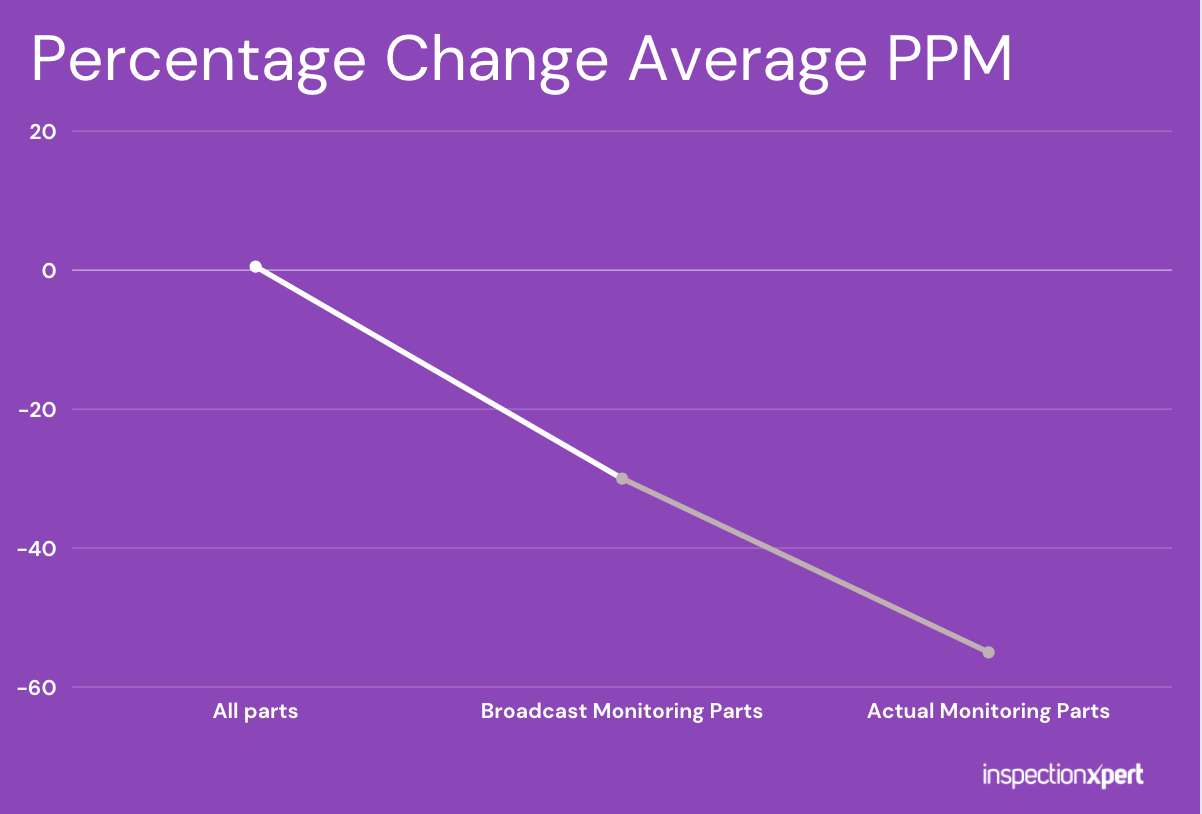
Invest In Quality, Because You Can’t Afford Not To
The key take-aways from this article are many, but they can all be summed up with this simple plan:
What does it really take to start down this path and see results in the form of ship-ready reports?
The New Normal: Efficient Quality Processes
The month-end ship list reveals a daunting list of deliveries for existing parts, new parts and revision change parts. A QA employee accesses a shared folder system and prints or exports files to send the customer. Internal parties are informed that the reports are released and the parts may be released for shipment. This occurred because print requirements became written specifications minutes after the drawing was imported, field headers were instantly updated, and humans kept their hands out of the day to day process.
Whether you are implementing to improve systems within your own four walls, or as a tool for better supplier management, InspectionXpert provides the foundation for easily and efficiently unifying your QMS documentation. If that sounds too good to be true, I assure you it’s not. And this isn’t an advertisement for InspectionXpert. I’ve used InspectionXpert at five different companies, and it’s the tool that has made me successful at them all.
About the Author:
Richard Schneider is the CEO of All Things Quality Inc. where he partners with job shops to help them create predictable, reliable and efficient ways to document customer and regulatory requirements, so that manufacturers can become the preferred supplier among their customers.
Richard holds a degree in Business Administration, has multiple ASQ certifications, and is an advanced level Zeiss Calypso CMM Programmer. If you would like to reach him for comment or consultation, he can be reached at rjohnschneider@hotmail.com, or (612) 414-4629.
Copyright 2026 Ideagen, All Rights Reserved